Keratin is a household of structural, fibrous proteins. These proteins are the first elements of hair, nails, feathers, claws, hooves, and the outer layer of pores and skin in animals.
There are two principal varieties: alpha-keratin and beta-keratin. Each are present in birds, however the beta-keratin is particular to birds (and reptiles) and is chargeable for most of the distinctive properties of birds.
Particularly, many elements of a fowl’s physique (feathers, beaks, and claws) are composed of each alpha-keratin and beta-keratin, and the person combination of those two keratin varieties is partly chargeable for the large selection in these physique elements.
Alpha-keratins are much less widespread in birds than in mammals. Chemically, these are proteins (giant molecules made up of amino acids joined in lengthy chains) that kind coiled, versatile buildings.
In distinction, beta-keratins are smaller proteins that kind inflexible, stacked sheets. This makes physique elements containing a big proportion of beta-keratins notably robust and sturdy.
One subfamily of beta-keratins is the feather-beta-keratin subfamily. Chemically, these keratins have some distinct traits leading to distinct materials properties:
- They’re comparatively small proteins of solely about 100 amino acids
- They comprise a core area of about 34 amino acids that’s extremely constant throughout totally different beta-keratins. This core is wealthy in two amino acids, glycine and tyrosine, that are a significant motive for the inflexible construction.
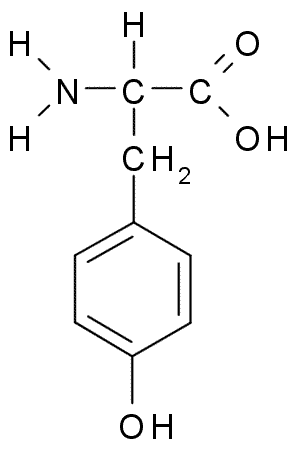
Glycine
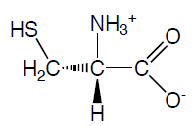
Tyrosine
- As talked about above, the polypeptide chains of beta-keratins are organized into beta-pleated sheets. These sheets are fashioned by hydrogen bonds between the carbonyl oxygen of 1 peptide bond and the amide hydrogen of one other, making a extremely secure, folded construction. The sheets then stack collectively to kind sturdy filaments.
- The stacked beta-pleated sheets are additional strengthened by disulfide bridges fashioned between cysteine models (cysteine is one other amino acid). These covalent bonds hyperlink adjoining protein chains, making a extremely cross-linked, inflexible, and insoluble materials. The excessive density of those disulfide bonds is a key issue within the larger toughness and chemical resistance of avian beta-keratin in comparison with mammalian alpha-keratin.
Cysteine
- The chemical nature of beta-keratin additionally contributes to the structural coloration of many fowl feathers. The protein self-assembles into intricate nanostructures inside feather cells. These buildings, together with air cavities, coherently scatter mild, producing vibrant, non-iridescent colors like blues and greens. This course of is distinct from pigment-based coloration and is instantly depending on the exact association of the keratin and air. It thus requires a extremely common construction that’s supplied by the beta-keratin.
Curiously, whereas feathered non-avian dinosaurs seemingly produced avian beta-keratin, they lacked specialised feather beta-keratin. This keratin has a better elasticity and thus is extra suited to flight feathers. Thus, this specialised beta-keratin could have performed a task within the growth of flight.
Illustrations: